Describe All the Methods Used in the Preparation of Esters
In the presence of protic acids sulphuric acid alcohols undergo dehydration to produce alkenes and ethers under different conditions. After the oxidation of primary and secondary alcohols we can get both aldehydes and ketones.
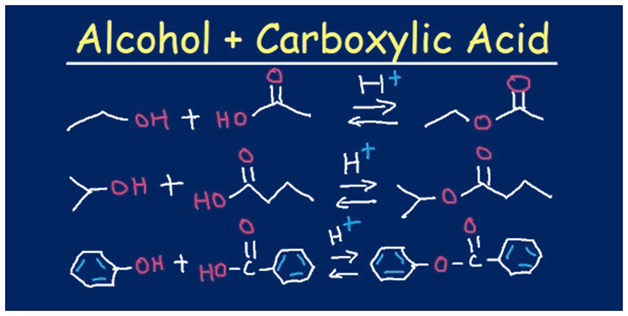
Formation Of Esters Mechanism Of Synthesis Of Esters Examples
Preparation of ethyl acetate ester.
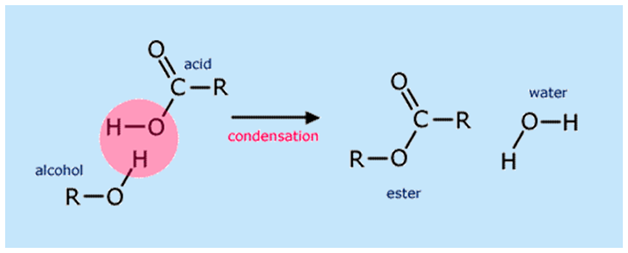
. Oxidation is a direct method most commonly used for preparation of carboxylic acids. The catalyst is usually concentrated sulphuric acid. Based on the method used select the necessary reactants that will produce your chosen ester.
Dispose all of the ester products in the organic waste container. Direct oxidation of alcohols ketons. WORK IN THE HOOD.
Like many organic compounds esters can be reduced in two ways. Synthesis or preparation of esters in the laboratory involves 3 steps. Table view List view Synthesis of Esters Reactant acid anhydride Reactant 2 alcohol Ester Chosen Preparation Used Choose.
Choose Choose From acid ai butyric anhy Choose. Anti-bumping granules are added to ensure a smooth boiling action. Aldehydes alkenes and alkyl benzene results in formation of carboxylic acids.
In this practical students explore the formation of esters through the ability of different alcohols to react with organic acids. 5 cm 3 ethanol 6 cm 3 butanoic acid. H 2 SO 4 or HC1g ester water are produced.
Table view List view Synthesis of Esters Ester Chosen Preparation Used Reactant 1 acid or anhydride Reactant 2 alcohol Choose. 1-octyl acetate Choose From acid Choose acetic acid Choose. This investigation is largely based around the preparation of esters and the factors that affect the yield of the ester.
Dry hydrogen chloride gas is used in some cases but these tend to involve aromatic esters ones where the. Together the class can quickly produce a range of esters on a test tube scale with a variety of interesting smells to sample and compare. As discussed in the above topic Preparation of carboxylic acid is possible from the usual strong oxidizing agents.
Based on the method used select the necessary reactants that will produce your chosen ester. Some esters can be prepared by esterification. Pour about 150 ml of tap water in a 250 ml beaker.
Esters are used as plasticizers. Wash the counters at the end of lab. Therefore it is preferable to use neutral or alkaline agents such as potassium permanganate for this preparation method.
In this reaction H 2 SO 4 or HCl are used as the dehydrating agent. This equilibrium can be displaced in favour of the ester by the use of. In the presence of sulphuric acid dehydration of ethanol at 443 K yields ethene whereas it yields ethoxyethane at 413 K.
Isobutyl alcohol 1 Choose. The drawback in this method is that most of the excess reactant is left unreacted requiring extra steps to remove it. Carboxylic acid were used the equilibrium would be driven towards ester product and for unhindered systems would result in a 90 yield.
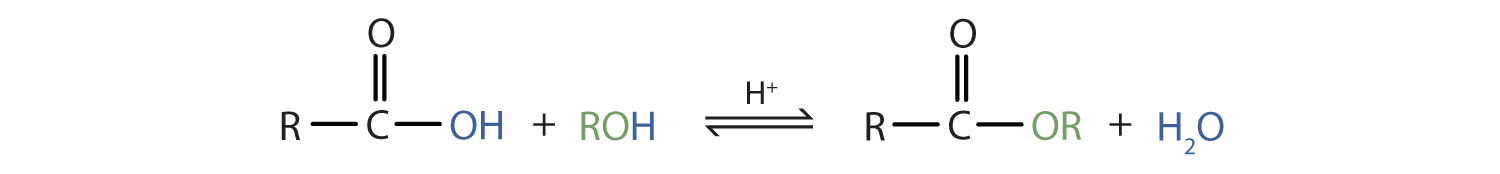
The reaction is reversible. Choose amyl acetate - Choose Choose. A reaction in which a carboxylic acid and an alcohol heated in the presence of a mineral acid catalyst form an ester and water.
Spills should be cleaned immediately. Esters are used for making artificial flavours and essences. From acid Choose acetic acid Choose amyl alcohol 2 Choose.
Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. They are formed when a carboxylic acid reacts with alcohol and a strong acid such as a catalyst called sulfuric acid HOSTS for this lab. STAGE 1 Making the ester.
By Oxidation of Alcohol. Place the test tubes in the water and heat the water on a hot plate to a temperature of about 60C 75 Leave the test tubes in the hot water bath for 15 minutes. Add 10 drops of concentrated sulphuric acid as a catalyst and gently swirl the contents in the flask.
Identify and describe the substances from which most esters are prepared. From acid Choose formic acid Choose. In either case the ester is cleaved to yield in addition to the alcohol or phenol from which it was derived a primary alcohol corresponding to the acid portion of the ester.
The structural formula for esters can be represented as R-COO-R. Add 25 cm 3 of ethanol to the flask. Synthesis of the ester Step 2.
The -OH group of carboxylic acid takes part in water formation not the -OH of alcohol. If carboxylic acid alcohol are heated with conc. Add 4 drops of concentrated sulphuric acid to each test tube.
There are various methods that can be used to prepare aldehydes depending upon the type and requirement of the compounds. In the round-bottomed flask the alcohol ethanol is mixed with the carboxylic acid ethanoic acid and a small amount of concentrated sulfuric acid catalyst is added too. The boiling point of ethyl ethanoate is 77C.
Preparation of Ethers by Dehydration of Alcohols. Heating Gem Dicarboxylic Acids. In practice often a 101 or 110 ratio is used which results in even higher yields of ester.
The reaction is reversible. From acid by Esterification. Isolation of the ester Step 3.
Preparation of Esters Introduction Esters are known for their pleasant smells such as perfumes and artificial flavorings in contained labs. Synthesis of Esters Ester Chosen Preparation Used Reactant 1 acid or anhydride Reactant 2 alcohol Choose. Esters are used as solvents for oils fats gums resins cellulose paints varnishes etc.
Add 25 cm 3 of ethanoic acid to the flask. Wear goggles at all times. The methods of changing the position of the equilibrium are well known.
As a class experiment this can be organised if desired as a class-cooperative investigation of the ability of a range of. The formation of an ester from a carboxylic acid and an alcohol. Following are some important methods of preparation of aldehydes.
A by catalytic hydrogenation using molecular hydrogen or B chemical reduction. Carboxylic acids formation is possible with mild oxidizing agents such as Tollen. Purification of the ester Esters of the low molar mass alkanoic acids and alkanols have fragrant fruity odours and.
Each preparation is potentially quite time consuming and technically. Concentrated sulfuric acid will burn the eyes and skin. Hydrolysis of acyl halides and anhydrides.
The direct method for the preparation of esters is the reaction between the carboxylic acid and the alcohol For example the ethyl acetate ester is obtained by the reaction of acetic acid and ethyl alcohol. Warm the mixture in a warm bath for 15 minutes. These are used in cold drinks ice-creams sweets and perfumes.
Ask your instructor for assistance.

Formation Of Esters Mechanism Of Synthesis Of Esters Examples
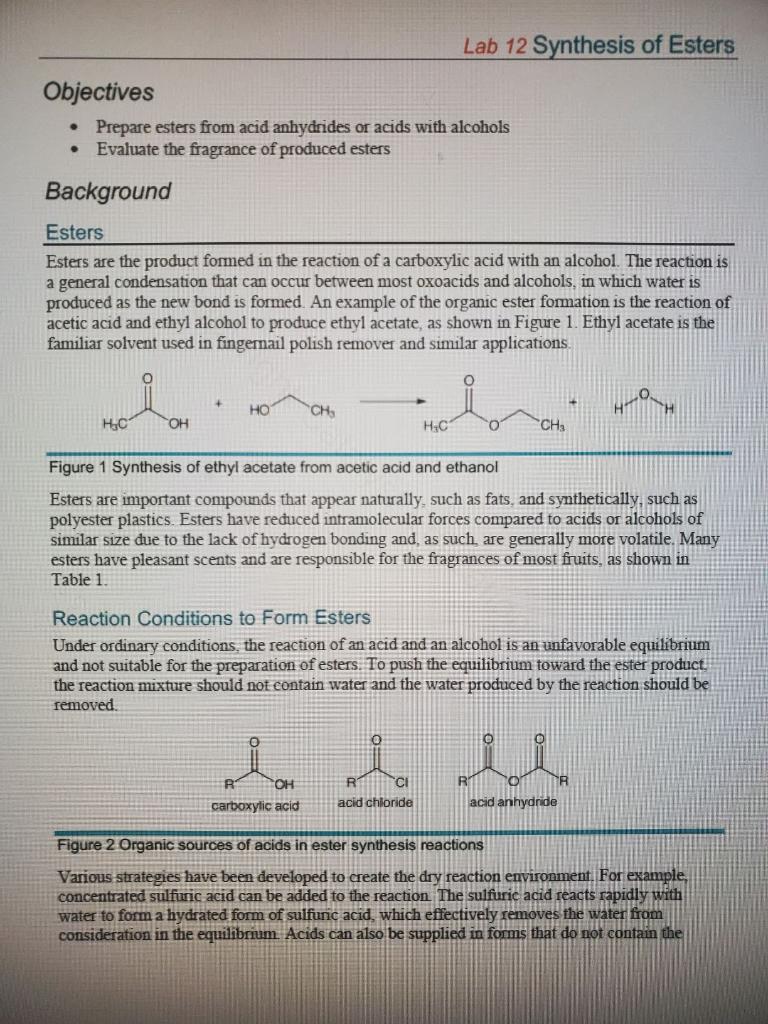
Solved Sample Data Esters Data 1 Esters Chosen For Chegg Com
No comments for "Describe All the Methods Used in the Preparation of Esters"
Post a Comment